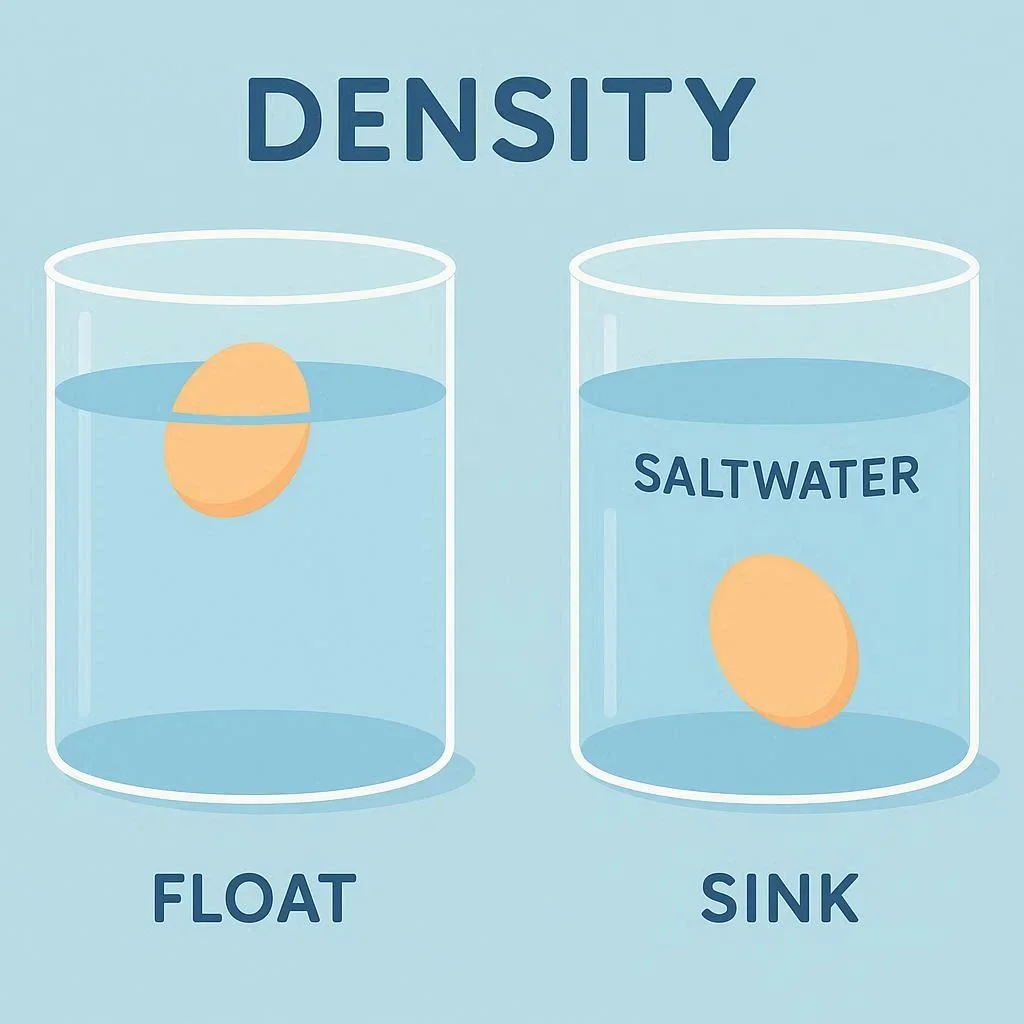
The Floating Egg Experiment is a classic, hands‑on demonstration that makes the abstract concept of density concrete for students of all ages. By simply watching an egg either sink or float in a glass of water, learners instantly grasp why some objects are heavier than others despite appearing similar. This simple physics experiment requires only everyday kitchen items, yet it opens a gateway to discussions about water density, buoyancy, and the science of solutions. In this article we will walk through the materials, step‑by‑step procedure, and the underlying science, giving teachers and curious parents a reliable density demonstration that can be repeated again and again.
Understanding Density in Everyday Life
Density is defined as mass per unit volume, a relationship that determines whether an object will sink or float when placed in a fluid. When an egg is placed in plain tap water, it typically sinks because its average density exceeds that of water (about 1 gram per cubic centimeter). Adding salt to the water increases the water’s density, allowing the same egg to float. This shift illustrates the principle of egg buoyancy and provides a vivid, observable example of how solutions can alter physical properties. For a deeper dive into the science of density, the Density article on Wikipedia offers a thorough explanation.
Materials Needed for the Experiment
- One fresh chicken egg (room temperature)
- Two clear glasses or beakers (250 ml capacity each)
- Tap water
- Table salt (non‑iodized works best)
- Stirring spoon
- Measuring spoon (optional for consistency)
All of these items are inexpensive and typically found in a home or classroom pantry, making the experiment accessible for a wide audience.
Step‑by‑Step Procedure
- Fill the first glass halfway with plain tap water.
- Gently place the egg into the water. Observe that it sinks to the bottom.
- In the second glass, fill it with the same amount of water.
- Add one tablespoon of salt to the second glass and stir until fully dissolved.
- Carefully lower the same egg into the salted water. Watch as the egg begins to rise and eventually floats.
- For extra exploration, gradually increase the salt concentration and record the point at which the egg just begins to float.
This procedure not only shows the effect of density changes but also encourages students to hypothesize, measure, and record data—key components of any science classroom activity.
Why the Egg Floats – The Science Explained
When salt dissolves in water, the solution’s mass increases while its volume changes only slightly, raising its overall density. The floating egg test demonstrates that the egg’s density is now lower than that of the salty water, causing it to experience an upward buoyant force equal to the weight of the displaced fluid (as described by Archimedes’ principle). The larger the difference between the egg’s density and the surrounding fluid, the stronger the buoyant force. This exploration ties directly into curriculum standards for middle school science, where students learn about Buoyancy and its real‑world applications.
Variations and Classroom Extensions
To extend the lesson, consider the following variations:
- Different liquids: Test the egg in sugar water, corn syrup, or oil to compare densities.
- Temperature effects: Warm water expands slightly, reducing its density; cool water contracts, increasing density. Students can investigate how temperature influences floating.
- Measurement challenge: Have students measure how many grams of salt are needed for the egg to just float, reinforcing data‑collection skills.
- Cross‑disciplinary links: Connect the experiment to mathematics by graphing salt concentration versus floating height.
Teachers can find ready‑made lesson plans and safety guidelines on the Science Buddies floating egg experiment page, which provides additional ideas for homework assignments and assessment rubrics.
Ensuring Success and Safety
While the materials are safe, always supervise younger children to prevent accidental ingestion of large amounts of salt. Encourage gentle handling of the egg to avoid cracks that could skew results. Clean up spills promptly to prevent slippery floors.
Conclusion
The Floating Egg Experiment transforms a simple kitchen activity into a powerful teaching tool for density, buoyancy, and scientific inquiry. By following the steps outlined above, educators can deliver an engaging, standards‑aligned lesson that sparks curiosity and reinforces core concepts. Ready to bring this hands‑on activity into your classroom or home? Download our free printable worksheet now and start exploring the wonders of density today!
Frequently Asked Questions
Q1. What materials are needed for the Floating Egg Experiment?
You need a fresh egg, two clear glasses, tap water, table salt, a spoon for stirring, and optionally a measuring spoon. All items are common household or classroom supplies.
Q2. Why does the egg sink in plain water but float in salty water?
Plain water’s density (~1 g/cm³) is lower than the egg’s average density, so the egg sinks. Adding salt increases the water’s density, making it heavier than the egg, which causes the egg to float due to buoyancy.
Q3. How much salt should I add to make the egg just float?
Start with one tablespoon of salt in about 250 ml of water and stir until dissolved. If the egg still sinks, gradually add more salt, noting the amount when the egg begins to rise. The exact amount varies with water volume and egg size.
Q4. Can I use other liquids instead of salty water?
Yes. Sugary solutions, corn syrup, or oil have higher densities and can also make the egg float. Testing different liquids demonstrates how density changes affect buoyancy.
Q5. What curriculum standards does the Floating Egg Experiment support?
The activity aligns with middle‑school science standards on density, buoyancy, and Archimedes’ principle. It also supports math integration through data collection, graphing, and measurement skills.