Floating Egg Experiments are a simple yet powerful way to explore the principles of density and buoyancy. By observing how a raw egg behaves in different liquids, students and curious minds can witness firsthand how the mass of an object and the volume of the fluid it displaces determine whether it sinks or floats. This classic experiment not only reinforces textbook concepts but also encourages hands‑on learning and critical thinking.
Floating Egg Experiments: Setting Up the Apparatus
Before you begin, gather the following materials: a raw egg, a clear glass or plastic container, tap water, salt, vinegar, and a measuring cup. The container should be tall enough to allow the egg to move freely without touching the sides. A small spoon or stir stick will help you gently place the egg in the liquid. For safety, wear a lab coat or apron if you’re working with vinegar or other chemicals.
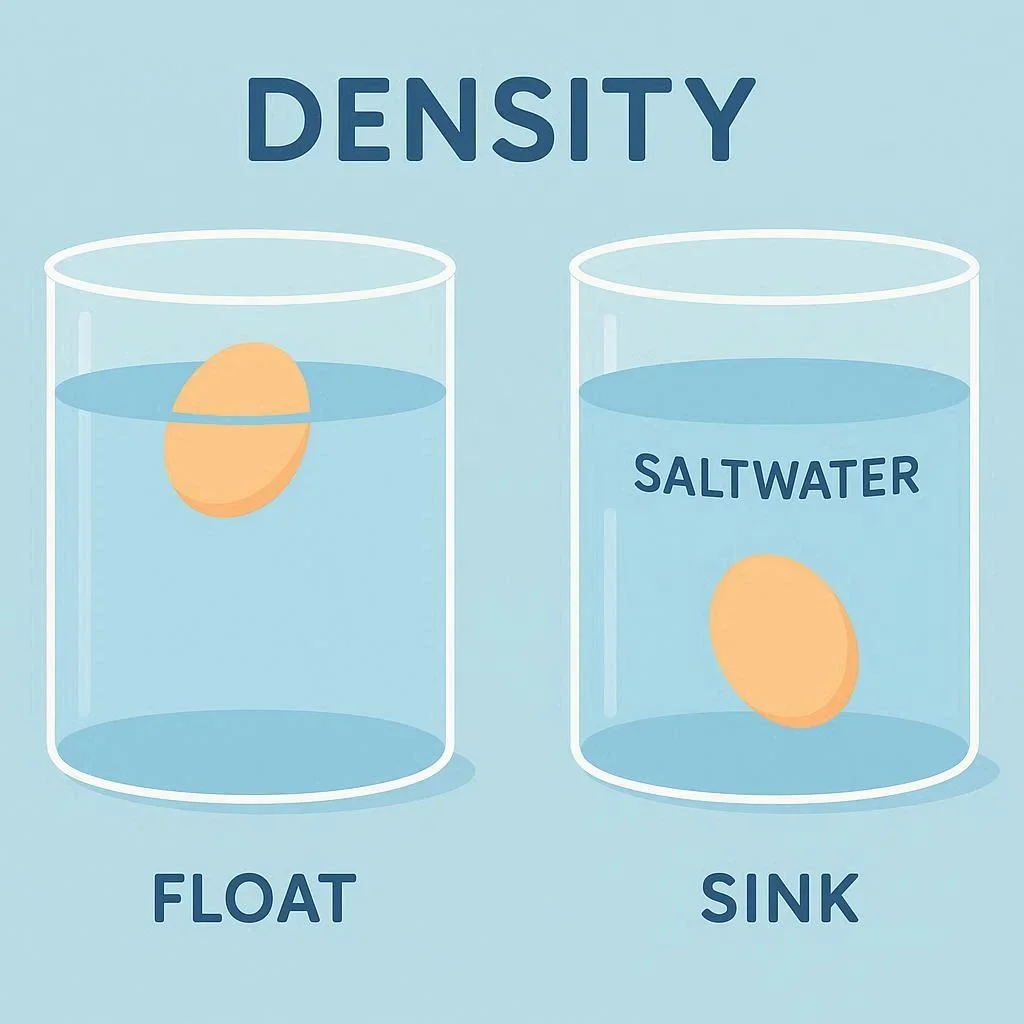
Start with plain tap water. Fill the container about three‑quarters full and place the egg gently inside. Observe whether it sinks to the bottom or floats. Most raw eggs will sink in tap water because their density is slightly higher than that of water. This baseline result sets the stage for the next steps of the experiment.
Floating Egg Experiments: Adding Salt to Increase Density
To make the egg float, you need to increase the density of the liquid. Dissolve salt in the water until the solution becomes denser than the egg. A practical rule of thumb is to add about 1 cup of table salt per 2 cups of water, stirring until fully dissolved. The salt ions replace some of the water molecules, raising the overall mass per unit volume.
Once the saltwater is ready, carefully lower the egg into the solution. If the egg rises to the surface and remains there, you’ve successfully created a denser medium. This demonstrates the principle of buoyancy: an object will float if its average density is less than that of the surrounding fluid.
Floating Egg Experiments: Using Vinegar to Test Density Changes
Vinegar offers a contrasting approach. Instead of increasing the liquid’s density, vinegar can dissolve the eggshell, allowing the egg’s contents to mix with the liquid. Fill the container with vinegar and place the egg inside. Over time, the shell will dissolve, and the egg’s yolk and white will disperse, creating a homogeneous mixture.
After the shell has dissolved, the egg’s contents will have a density similar to the vinegar, causing the mixture to float. This part of the experiment illustrates how chemical reactions can alter physical properties, providing a deeper understanding of density changes in real‑time.
Floating Egg Experiments: Recording Data and Drawing Conclusions
To make the experiment scientifically rigorous, record the following data: the volume of liquid used, the amount of salt added, the time taken for the egg to sink or float, and any observable changes in the egg’s appearance. Use a ruler to measure the height of the egg above the bottom of the container when it floats.
Compile your observations in a table:
- Plain water – Egg sinks (average density 1.03 g/cm³)
- Saltwater (1 cup salt per 2 cups water) – Egg floats (density ~1.05 g/cm³)
- Vinegar – Egg dissolves, mixture floats (density ~1.01 g/cm³)
These results confirm that density is a key factor in buoyancy. By manipulating the liquid’s density, you can control whether an egg sinks or floats. This experiment also highlights the importance of precise measurement and careful observation in scientific inquiry.
Floating Egg Experiments: Extending the Lesson with Advanced Concepts
For students ready to dive deeper, introduce the concept of Archimedes’ principle. Explain that the upward buoyant force equals the weight of the fluid displaced by the egg. Use the formula Fb = ρfluid × Vdisplaced × g to calculate the force and compare it to the egg’s weight.
Another extension involves exploring temperature effects. Warm water has a lower density than cold water, so an egg that floats in cold water may sink when the water is heated. This demonstrates how temperature can influence density and, consequently, buoyancy.
Conclusion: Why Floating Egg Experiments Matter
Floating Egg Experiments provide a tangible, engaging way to explore fundamental physics concepts. They reinforce the relationship between density, buoyancy, and fluid mechanics while encouraging curiosity and experimentation. By recording data, analyzing results, and extending the lesson, learners gain a comprehensive understanding of how everyday materials behave under scientific scrutiny.
Ready to bring science to life? Start your own Floating Egg Experiments today and watch the mysteries of density unfold right before your eyes!
For more detailed explanations on density and buoyancy, visit Wikipedia: Density, NASA: Fluid Dynamics Experiments, Khan Academy: Forces and Motion, and National Geographic: Egg Buoyancy.
Frequently Asked Questions
Q1. Why does a raw egg sink in tap water but float in saltwater?
A raw egg sinks in tap water because its average density (~1.03 g/cm³) is slightly higher than that of water (~1.00 g/cm³). Adding salt increases the water’s density, making it greater than the egg’s density, so the egg floats. This demonstrates the principle that an object will float if its density is less than the surrounding fluid’s density.
Q2. Can I use other liquids besides saltwater and vinegar for this experiment?
Yes, you can try sugar water, oil, or even milk. Each liquid has a different density, so by adjusting the concentration you can observe whether the egg sinks or floats. This variation helps illustrate how density changes with solute concentration.
Q3. What safety precautions should I take when using vinegar?
Vinegar is mildly acidic, so wear a lab coat or apron to protect clothing. Keep the experiment away from children’s reach and avoid inhaling fumes in a poorly ventilated area. Dispose of the vinegar solution responsibly after the experiment.
Q4. How does temperature affect the floating egg experiment?
Warm water has a lower density than cold water because it expands. An egg that floats in cold water may sink when the water is heated, showing that temperature can influence buoyancy by changing fluid density.
Q5. How can I calculate the buoyant force acting on the egg?
You can use Archimedes’ principle: F_b = ρ_fluid × V_displaced × g. Measure the volume of the egg, the density of the fluid, and use g = 9.81 m/s² to compute the upward force and compare it to the egg’s weight.